Which of the Following Molecules Contains a Polar Covalent Bond
304 - 265 039. H2O and HCl are polar covalent.

Solved 5 Which Of The Following Molecules Contains A Polar Chegg Com
Water mathrmH_2 mathrmO text b.

. Beginarrayltext a. The main type of interaction between molecules of hydrogen H2 are polar covalent. You must draw Lewis structures and determine shape before answering 1st attempt Choose one or more.
Dipole moment is a vector quantity. But in hetero atoms different atoms or hetero nuclear molecules like H C l H F etc. The following molecules contain polar covalent bonds.
Which of the following molecules contains the strongest polar covalent bond. O CC14 O CHCI3 O BH3 O H20 O CO2. The following molecules all contain polar bonds.
Which of them are polar molecules. H2 contains the strongest polar covalent bond. For symmetrically applicable molecular dipole moment is 0.
A covalent bond is formed when. The P contains four areas of electron density around it three areas bonding and one area non-bonding lone pair. Sulfur mathrmS_8 text b.
When a covalent bond is formed between homo atoms similar atoms like C l 2 O 2 etc. Which of the following molecules contain polar covalent bonds. Fluorine mathrmF_2 text d.
Thus the polar bonds are arranged non- symmetrically in a trigonal pyramidal molecular geometry. HCl H2O CO2 NH3. Dipole moment is zero for non-polar molecules.
Fluorine mathrmF_2 text c. а со S02 CCIA H2O В СНcia. Choose all that apply.
316 - 096 22 Non-covalent C. Which of the following molecules contains a covalent bond. 40 - 21 19 Covalent B.
Bonds which have large electronegativity difference like HCl have partially ionic and partially covalent character called polar covalent bonds. Which of the following moleculesspecies have identical bond order and same magnetic properties. All of the above.
Hydrogen bromide HBr endarray. The following molecules contain polar covalent bonds. A CHCI B CHCl C CHC D CC E None of these choices Which compound is a ketone.
D CHF E None of these choices B LiF C F. Which of the following elements has six valence electrons. Carbon tetrachloride has zero dipole moment since the.
20 and it only involves nonmetal then the molecule contains a covalent bond. Carbon dioxide being linear the net bond moment is equal to zero since the individual bond moment cancel with each other. Nitrogen mathrmN_2endarray.
Which of the following compounds contains polar covalent bonds. E None of these choices. Which are overall nonpolar molecules.
Which molecule is the only nonpolar one. Choose one or more. Then the sharing electrons is unequal and polar bonds are formed.
The following molecules contain polar covalent bonds. Which of the following molecules contains a polar covalent bond. Helpful 0 Not Helpful 1 Add a Comment.
Which of the following molecules contains a polar covalent bond. Use the electronegativity values from the periodic table link to help you answer this - 1399622. So it is non polar.
The concept of a chemical bond is _____. Which are overall nonpolar molecules. How two or more atoms are held together.
The following molecules contain polar covalent bonds. So the correct answer is hydrogen peroxide. There are three sigma bonds present in hydrogen peroxide.
Nitrogen molecule is a non polar covalent compound. Which of the following compounds has polar covalent bonds. Two of them are between hydrogen and oxygen atoms which is polar in nature.
Iodine monochloride ICl text d. LiI-- thats ionic- so is not molecular under normal conditions- if a molecule were formed in gas phase then that would be polar too. Phosphorous tribromide contains polar bonds.
Which molecule does not have a dipole moment. Which molccule has a zero dipole moment. Which of the following molecules contain polar covalent bonds.
A H2 B O2 C CO2 D H2O E CH4. H2 has non polar covalent bond. Which of the following molecules contains a.
Carbon monoxide CO text c. Then equal sharing of electrons occurs and a nonpolar bond is formed. Since the Cl atom is much more electronegative than the hydrogen atom the covalent bond between two atoms is quite polar.
Beginarrayltext a. The other one is between two oxygen atoms where there is no unequal sharing of electrons as they have same dipole moment.
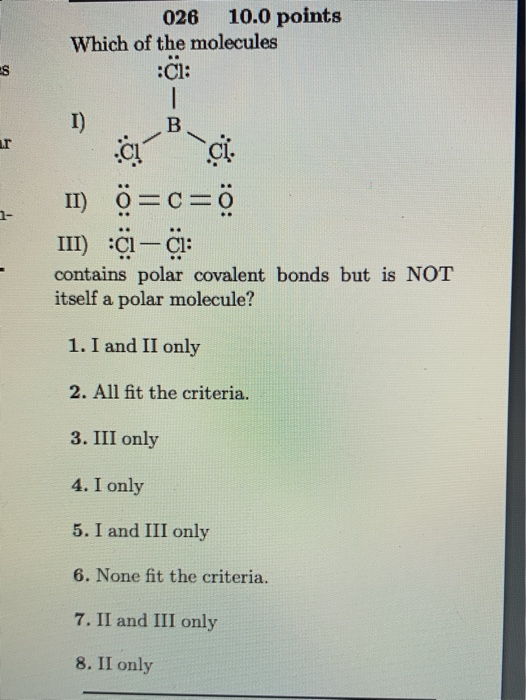
Solved Es Ur 026 10 0 Points Which Of The Molecules C1 Chegg Com

Polar Covalent Bond Definition And Examples

Covalent Bond Polar Covalent Bond Non Polar Covalent Bond Examples By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
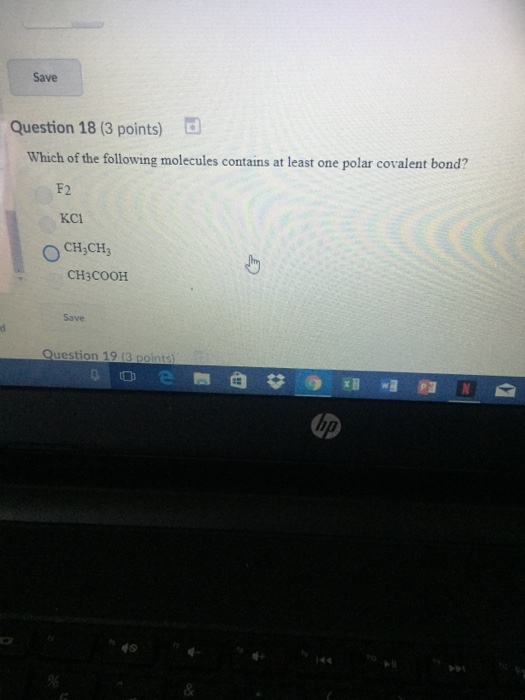
Solved Which Of The Following Molecules Contains At Least Chegg Com
No comments for "Which of the Following Molecules Contains a Polar Covalent Bond"
Post a Comment